Abstract
Background: BAX 335 is an adeno-associated virus serotype 8 (AAV8)-based clotting factor IX (FIX) Padua (R338L) gene therapy for the treatment of hemophilia B. A phase 1/2 safety and efficacy study dosed 8 participants with BAX 335 between 2013 and 2015 (NCT01687608). Infusions with BAX 335 were generally well tolerated; however, 7 of 8 participants did not achieve significant levels of durable transgene expression despite the use of glucocorticoids and resumed FIX replacement by 52 weeks (Konkle et al, Blood 2021;137:763-74). A root-cause analysis identified CpG content in the vector as a potential immunogenic trigger for the innate immune response, resulting in loss of expression. One participant demonstrated high levels of durable FIX expression, with detectable FIX activity levels between 0.1 and 0.4 IU/mL after 4 years and was found to have a missense variant in the IL6R gene. This study is the longest ongoing clinical gene therapy trial using a FIX R338L transgene.
Aims: (1) To report long-term safety and participant disposition; (2) to describe persistence of endogenous FIX R338L transgene expression; and (3) to report long term persistence of neutralizing anti-capsid antibodies.
Methods: Eight adult male participants with hemophilia B (FIX activity ≤2%) received BAX 335 in 1 of 3 intravenous dose cohorts (2.0 x 1011, 1.0 x 1012, or 3.0 x 1012 vector genomes/kg). The primary study objective was to assess the safety of BAX 335. Secondary assessments included the kinetics of plasma FIX activity, the need for exogenous FIX replacement, and systemic immune responses to BAX 335 and vector transgene protein. FIX clotting activity was measured using the one-stage clotting assay at the central laboratory. Serum neutralizing antibodies against the AAV8 and AAV2 capsids were measured to Year 5 following BAX 335 infusion using an in vitro transduction inhibition assay. Laboratory and in-person assessments were conducted for the first 5 years after BAX 335 infusion (one unscheduled visit was made at 7.2 Years). This was followed by annual liver ultrasound scans and telephone questionnaires assessing health status with the aim of completing 15 years of follow-up per participant. The study was approved by the institutional review boards of all participating sites, and all participants provided written informed consent.
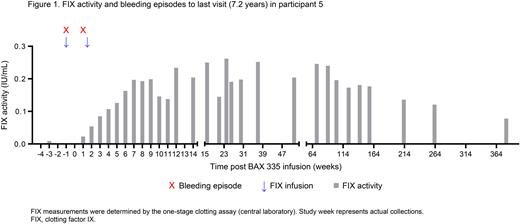
Results: Of 8 participants enrolled (aged 20-69 years), 2 discontinued from the study approximately 1 and 3 years after receiving BAX 335 infusion, and another participant withdrew consent 2 weeks prior to the data cutoff of May 6, 2022. Data from the 6 participants who had >5 years of long-term safety follow-up were included in this analysis. At data cutoff, 176 adverse events (AEs) were reported in all 8 dosed participants, most (72%) occurred in Years 1 and 2. In total, 6 serious adverse events (SAEs) were reported in 3 participants, of these 2 SAEs (post-procedural hemorrhage, arthritis) were reported after the interim analysis reported by Konkle et al. 2021. None were considered to be related to BAX 335 and all resolved. Although long-term hemostatic protection from bleeds was not observed in most of the participants in this analysis, 1 participant had continued FIX activity (Figure 1), with a most recent value of 0.078 IU/mL at 7.2 years after BAX 335 infusion. This participant has neither experienced any bleeds nor needed FIX replacement therapy after the second week of vector infusion. No FIX inhibitors have been observed. At Year 5, AAV neutralizing antibodies persisted at high titers in most participants (reciprocal of serum dilution: AAV2, <5 to 10,240; AAV8, 80 to 40,960).
Conclusions: No additional BAX 335-related AEs, malignancy or thrombosis were reported in this long-term follow-up analysis of a phase 1/2 study. One participant achieved persistent FIX transgene activity in the circulation for 7.2 years and remains free from bleeding and the need for FIX replacement therapy. This is the longest duration of FIX R338L expression reported. Consistent with other studies, AAV neutralizing antibodies remained persistent at titers that would limit re-dosing with current AAV platforms. Future studies should explore patient-specific factors that may predict persistent transgene expression.
Disclosures
Escobar:Novo Nordisk: Honoraria; Takeda: Honoraria; Bayer: Honoraria; CSL Behring: Honoraria; Genentech: Honoraria; UniQure: Honoraria; Kedrion: Honoraria; Hemobiologics/LFB: Honoraria; The National Hemophilia Foundation: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria; BioMarin: Honoraria. Konkle:Takeda: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sigilon: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Baxalta: Research Funding; CSL Behring: Honoraria; Spark: Honoraria; Uniqure: Honoraria, Research Funding; BioMarin: Honoraria. Ducore:Bayer: Honoraria. Rajavel:Takeda: Current Employment, Current equity holder in publicly-traded company. Sun:Takeda: Current Employment, Current equity holder in publicly-traded company. Yan:Takeda: Current Employment, Current equity holder in publicly-traded company. Chapin:Takeda: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal